I’m about to go to the 2 hour Point of Inquiry interview of Richard Dawkins. Could be interesting.
(This is a syndicated post. Read the original at FreeThoughtBlogs.)
I’m about to go to the 2 hour Point of Inquiry interview of Richard Dawkins. Could be interesting.
(This is a syndicated post. Read the original at FreeThoughtBlogs.)
A great moment. At the secularism panel just now with Barry Kosmin and Ron Lindsay and Phil Zuckerman, moderated by Paul Fidalgo, Paul asked the audience, how many of you have attended a Secular Sunday Assembly?
A LOT of hands went up.
Ron said something I didn’t hear, and Paul said, “That’s a great question – how many of you have gone twice?”
One hand went up.
(This is a syndicated post. Read the original at FreeThoughtBlogs.)
Taslima took this action shot yesterday.

(This is a syndicated post. Read the original at FreeThoughtBlogs.)
Taslima tweeted yesterday evening –

(This is a syndicated post. Read the original at FreeThoughtBlogs.)
I got here.
Talked to all the people at the reception.
Dropped food on the floor.
Generally misbehaved.
(This is a syndicated post. Read the original at FreeThoughtBlogs.)
I upset quite a few people the other day with that Nail polish post. Some of the people who were upset are, frankly, assholes, and they can go ahead and be upset, but a lot of them aren’t, and I’m sorry I upset those people.
I didn’t like or agree with everything in that Elinor Burkett article, and I skipped over most of them – but maybe I should have said I wasn’t endorsing the whole thing.
Someone in a Facebook group recommended this tumblr post cis by default, and I found what it says resonates with me a lot. It starts with some body discomforts, and then moves to the social.
I also just had a lot of discomfort with the more social (not physical) aspects of gender. And this admittedly did start from a lot of conversations about trans and variant gender identities – partially because they made me realize that everyone else did not, in fact, think about gender the way I did. Hearing both cis and trans people talk about how they had this mental sense of gender confused me, because I never felt that way. I identified as female, yes, but only because I I had the traits that defined that category – the same way that I was seen as [mostly] white (another whole issue) or labelled as upper middle class, it was just a role that was assigned to me based on how society organizes categories. And I could deal with that.
That’s just it, you know? It’s a fact – that is, it’s what you’ve always been told. That doesn’t necessarily make it something you identify with. The more I read about this and have conversations about it, the less convinced I am that I’ve ever identified as being female – but I haven’t (mostly) rejected it either. It’s just there. I deal with it.
But when people talked about what it meant to them to identify as a woman, and things like that, I felt left out in the cold. Because for me, while I’m fine with the fact that I have certain physical traits, and thus fall into a certain category, if it comes down to that sense of “me” that makes my core mental personality…there’s nothing about being a “woman” there.
Yeah. There isn’t.
Or at least…not very much. Other things loom much larger.
On the other hand I do identify as a feminist. That’s one of the things that looms larger. And being a feminist does in a way cause me to identify as a woman more than I otherwise would. And that makes me think about what it would be like to be a woman if feminism didn’t exist…and I can’t wrap my head around it. Everything I try to think on the subject is itself feminist, so it breaks down. “If there were no feminism I…I…I would be frustrated and angry.” If there were no feminism what would I be frustrated and angry about? It’s hard to think about. At any rate the idea of being a woman with no feminism in existence is bleak.
But there have been women in that situation since forever, and there still are. Yes, but that’s a different world. I grew up in this one, and it’s what shaped me, and that one would be like air to a fish.
What if there were no feminism but there were trans people, and transitioning were totally mainstream and unproblematic?
I don’t know. I can’t tell. I can’t tell if what I would feel in that situation is gender dysphoria, or something much milder. I suspect it’s the latter, but I really don’t know.
So eventually, even though I had questioned myself for a long time, I ended up just staying as “cis” because it mostly worked, even if imperfectly; and for the most part I don’t usually bring up my discomforts unless it’s with people who I think are worth opening up to about it. I’m aware that I still have a privilege over trans people in many ways since for all intents and purposes, the world still sees me as cis. (Even though when I’ve had some bits of worse dysphoria, and craved to have someone see something other than “cis girl”, it’s never happened, and I’ve sort of just given up on that. Although it still makes me a bit happy when someone accidentally says “sir”, even if it’s a bit disappointing when they immediately correct themselves. But that’s still relatively minor compared to what other people have to deal with.)
It works for me too, but that’s because I have a lot of room to be eccentric. If I didn’t…I don’t know, I can’t even imagine how I would function then, because that would be a different person. I’m a cis by default weirdo; that’s my identity.
(This is a syndicated post. Read the original at FreeThoughtBlogs.)
The second one was the next day, after I’d re-read some Anne.
May 25, 2009
But physical punishment or ‘correction’ has been morally unproblematic until very recently, some of you retort.
I don’t buy it. I’m at least very skeptical. I agree that it’s been widespread – but not that it’s been morally unproblematic. Of course it was morally unproblematic to some people, to many people, but I’m claiming that to a substantial minority it was not. (I’m talking about the 19th century onwards, if only because there’s so much more literature for children and about children starting then. I could talk about Hogarth on cruelty – but I won’t, for now.)
After writing about Anne of Green Gables from memory I started wondering…wasn’t there a subsidiary character, who did recommend beating? That neighbor? Didn’t she say at some point ‘You ought to beat that child, that’s what’? In other words wasn’t the issue made explicit at some point – didn’t Marilla have a choice, which she made, for our edification?
So I re-read the first half or so. (Don’t scorn; it’s a good book; sentimental, yes, but not too cloyingly so, though I skip most of Anne’s long speeches about the fairies in the glen and whatnot – I’m as bored by them as Marilla is.) Yes, there is. Rachel Lynde comes up to Green Gables to meet Anne, and promptly points out how skinny and homely and red-haired she is, at which Anne loses her temper and shouts at her; Marilla rebukes her and sends her to her room. Mrs Lynde says to Marilla, among other things, ‘You’ll have your own troubles with that child. But if you’ll take my advice – which I suppose you won’t do, although I’ve brought up ten children and buried two – you’ll do that “talking to” you mention with a fair-sized birch switch.’ After she leaves Marilla wonders what she should do. ‘And how was she to punish her? The amiable suggestion of the birch switch – to the efficiency of which all of Mrs Rachel’s own children could have borne smarting testimony – did not appeal to Marilla. She did not believe she could whip a child. No, some other method must be found to bring Anne to a proper realization of the enormity of her offence.’
Well…why couldn’t Marilla whip a child? Or why did she not believe she could? Because she found it morally problematic. She’s a very unbending character, who conceals her affection for Anne for a long time, yet she can’t whip a child. This is apparently plausible, and not unreasonable, and in fact subtly admirable, in a very popular children’s book published in 1908. It can’t have been an extremely eccentric attitude. It wasn’t universal, but it wasn’t freakish, either.
(This is a syndicated post. Read the original at FreeThoughtBlogs.)
I was in a Facebook conversation earlier today with a friend who wanted to know if people recommended Anne of Green Gables, and later I remembered that I’d written at least one post on the subject. Actually there were two (I’m not counting one about a blasphemous cover for a new edition).
May 24, 2009
You know, I’ve been thinking. There’s this line the religious involved in the Irish nightmare have been giving us – this ‘we didn’t realize beating up children and terrorizing them and humiliating them was bad for them’ line. It’s Bill Donohue’s line too – ‘corporal punishment was not exactly unknown in many homes during these times, and this is doubly true when dealing with miscreants.’
You know what? That’s bullshit. I’ve been thinking about it, and it’s absolute bullshit. It is not true that in the past it was just normal to beat children, or that it was at least common and no big deal, or that nobody realized it was bad and harmful. That’s a crock of shit.
Think about it. Consider, for instance, Anne of Green Gables, published in 1908. Marilla doesn’t really want Anne at first, and she’s less charmed by her than Matthew is. She discourages Anne’s fantasies and her chatter, and she’s fairly strict – but she never beats her, and the thought doesn’t even cross her mind. If it were so normal to beat children – wouldn’t Marilla have given Anne a good paddling for one or more of her many enthusiastic mistakes? Wouldn’t she have at least considered it? But she doesn’t. Why? Because she’s all right. She’s a little rigid, at first, but she’s all right – she’s a mensch – she has good instincts and a good heart. She can’t be a person who would even think of beating Anne. Well why not? Because we wouldn’t like her if she did. So it’s not so normal and okay after all then. And this was 1908.
Think of Jane Eyre. There is beating and violence and cruelty to children there – Mrs Reed treats Jane abominably, and Lowood school (based on the Clergy Brothers School that Charlotte Bronte and her sisters attended) was very like Goldenbridge, complete with starvation and freezing and humiliation and beating. But it’s not okay! It’s not normal, it’s not just How Things Are – it’s terrible, and shocking, and wrong. Think of Mr Murdstone in David Copperfield – he’s not okay; he’s a very bad man. Think of Dotheboys Hall in Nicholas Nickleby – not okay. Think of the poor house in Oliver Twist – not okay. Think of the way Pap was always beating Huck Finn – not okay. Think of Uncle Myers in Mary McCarthy’s Memories of a Catholic Girlhood – very Goldenbridge; not okay.
I’m having a very hard time thinking of any classic fiction in which children are beaten or smacked and it’s treated as completely routine and acceptable. I don’t think that’s some random accident, I think it’s because most people have always known that it’s wrong to treat children like punching bags. Beating and other cruelty may have been much more common a few decades ago, but it was by no means universal, and it was not universally acceptable. So if you hear people peddling that line – tell them it’s a crock.
(This is a syndicated post. Read the original at FreeThoughtBlogs.)
I’m leaving tomorrow to go to the Reason for Change conference in Amherst (outside Buffalo). Things will be slow here.
If any of y’all have something you want to say in a guest post, send it to me in the next few hours and I’ll schedule it for while I’m gone (unless it’s no good, but how likely is that?).
Also, go to the conference!
Critical thinking is not an end in itself. It is a means to effect positive change, to transform our world for the better. At “Reason for Change,” the Center for Inquiry’s 2015 international conference, we’ll bring the skeptic and humanist communities together to do just that.
And we’ll do it in a place that many consider to be “home” to the skeptic and humanist movements: Western New York and CFI’s headquarters in Buffalo. Fittingly, 2015 will be the 35th anniversary of Free Inquiry and the 39th anniversary (last party before 40!) of Skeptical Inquirer, the two foundational publications that helped start it all.
This conference will be truly special. It will be both a celebration of our accomplishments and a robust examination of the challenges we still face. It will be an invaluable opportunity to connect and collaborate with thinkers, activists, researchers, and other luminaries from around the world. It will honor the individuals who have made transformative contributions to the advancement of science, reason, and free inquiry while also highlighting the next wave of up-and-coming activists.
That’s a great theme, and one I take a sharp interest in. It’s a meme among the little knot of people who make a hobby of complaining about me (and others) that I used to be skeptical and good but I’ve done a complete 180. Nope. I haven’t. I was a feminist then; I gave a shit about rights and equality and justice then. I’m very interested in how humanism and critical thinking intersect.
(This is a syndicated post. Read the original at FreeThoughtBlogs.)
Meanwhile, in another part of the primeval forest…
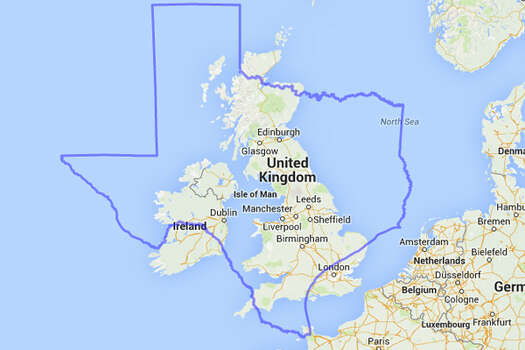
Federal appellate court to Texas women – Sorry, sucks to be you.
A federal appellate court upheld some of the toughest provisions of a Texas abortion law on Tuesday, putting about half of the state’s remaining abortion clinics at risk of permanently shutting their doors and leaving the nation’s second-most populous state with fewer than a dozen clinics across its more than 267,000 square miles. There were 41 when the law was passed.
Ten clinics, for a state bigger than France.
Bigger than Germany, bigger than the UK.
A three-judge panel of the appellate court, the United States Court of Appeals for the Fifth Circuit, in New Orleans, sided for the most part with Texas and the abortion law the Republican-dominated Legislature passed in 2013, known as House Bill 2.
The judges ruled that Texas can require all abortion clinics in the state to meet the same building, equipment and staffing standards that hospital-style surgical centers must meet, which could force numerous clinics to close, abortion rights advocates said.
In addition to the surgical standards, the court upheld a requirement that doctors performing abortions obtain admitting privileges at a hospital within 30 miles of a clinic. The court said that except as applied to one doctor working in McAllen in South Texas, the provision did not put an unconstitutional burden on women seeking abortions.
Because if those sluts need abortions they’d better damn well get out of rural Texas – and be prepared to wait in long lines, too. Serves them right.
Throughout the ruling, the Fifth Circuit judges cited the explanations given by the Texas Legislature for what is considered one of the most restrictive abortions laws in the country.
“Texas’ stated purpose for enacting H.B. 2 was to provide the highest quality of care to women seeking abortions and to protect the health and welfare of women seeking abortions,” the Fifth Circuit ruling read. “There is no question that this is a legitimate purpose that supports regulating physicians and the facilities in which they perform abortions.”
Said the court, blinking innocently.
The decision by the Fifth Circuit, regarded as one of the most conservative federal appellate courts in the country, is expected to take effect in about 22 days. In the meantime, however, the clinics and their lawyers plan to ask the court to stay the decision while they appeal it. If the Fifth Circuit declines, the clinic lawyers said, they will seek an emergency stay from the Supreme Court that would prevent the ruling from taking effect while the Supreme Court considered whether to hear the case.
Because this situation is a fucking emergency for women who desperately need to stop being pregnant. This isn’t some game. It’s people’s lives.
Lawyers for the Texas clinics that sued the state said about 900,000 reproductive-age women will live more than 150 miles from the nearest open facility in the state when the surgical-center requirement and admitting-privileges rule take effect.
The Fifth Circuit panel found that the percentage of affected women who would face travel distances of 150 miles or more amounted to 17 percent, a figure that it said was not a “large fraction.” An abortion regulation cannot be invalidated unless it imposes an undue burden on what the Supreme Court has termed “a large fraction of relevant cases.”
Oh I see – an undue burden is fine if it applies to “only” 17% of people.
Previously, a panel of the same federal appeals court ruled that Mississippi could not force its only remaining abortion clinic to close by arguing that women could always travel to neighboring states for the procedure. But the panel in the Texas case on Tuesday held that the closing of a clinic in El Paso — which left the nearest in-state clinic some 550 miles to the east — was permissible because many women had already been traveling to New Mexico for abortions, and because the rule did not close all the abortion clinics in Texas.
That’s the standard? It doesn’t close every last clinic in a state the size of Germany?
Oh well, it’s only women.
(This is a syndicated post. Read the original at FreeThoughtBlogs.)
Anne Perkins has some pleasingly acid thoughts on Tim Hunt FRS.
Here at last is someone who has come out with it. Women at work are a nuisance.
Hunt chose his moment of public revelation at, of all places, a women’s convention on science and journalism in South Korea. Perhaps he thought they’d be flattered when he told them that the trouble with women in labs was that they fall in love and cry when they’re criticised.
Of course they’d be flattered – he’s a Nobel laureate. He’s talking about them. What could be more flattering?
Note that old device, that get-out-of-jail-free admission of chauvinism.
These are not the words of a victim whose meal was spiked with a mysterious truth drug, they are the proudly admitted perceptions of a scientist. A scientist. Drink that in.
Yet, from his reaction, which was in the familiar non-apology apology of “I am sorry if I have caused offence, I should never have said such a thing in front of journalists”, it appears that he thinks it is he who has been in some way traduced, confounded by that dratted tendency of women not to get the joke. It seems quite likely that he is even now overwhelmed with supportive messages from colleagues for confronting the feminist thought police.
Talking of witch hunts (accompanied by “I promise I’m not making this up”) and The Shirt and locker room exploits and and and.
Even the response of the Royal Society suggests that the great institution doesn’t entirely get it. Science needs everyone regardless of gender, they said as they frantically pedalled away from one of their leading lights. How about, sexism is wrong, full stop?
Yeah. “We have to pander to these silly prejudices women have about being dismissed and belittled, because dammit we need their tiny little fingers, so keep it in mind next time old boy.”
What is both shocking and bewildering about Hunt’s jovial after-dinner remarks is that this is the considered view of someone whose life has been devoted to not taking the world for what it seems to be.
How bizarre that someone so entirely unreflective about his immediate surroundings can win a Nobel prize for original work. How bizarre that when he delivers his Nobel laureate lecture he describes (with a self-deprecation that is the luxury of an unchallenged inner sense of rectitude) the way that breakthroughs in his understanding came from mistakes, like running a centrifuge for too long or attributing unexpected results to contamination, but it never occurs to him to examine his own assumptions about the people with whom he works.
Yes and no. Mostly no, because they’re not really the same kinds of reflection, the same kinds of understanding, the same kinds of examination. But it would be nice if even scientists could learn some minimal social truths.
(This is a syndicated post. Read the original at FreeThoughtBlogs.)
First ever UK prosecution for forced marriage:
A 34-year-old Cardiff man has become the first person in the UK to be prosecuted under forced marriage laws introduced a year ago.
The man, who cannot be identified for legal reasons, was jailed for 16 years after admitting making a 25-year-old woman marry him under duress last year.
He also pleaded guilty to charges of rape, bigamy and voyeurism at Merthyr Crown Court.
The “under duress” sounds mild. The details are not mild.
The judge said the offences began when the woman became engaged last year and in March 2014 he took her to his house under the pretence of having a meal with his wife.
“Your house was empty, you locked the front door and drew the curtains, you ignored her pleas to let her go and threw her mobile phone away and bound and gagged her with scarves belonging to your wife,” he said.
“You tied her hands behind her back, she was bruised by the ties and she couldn’t breathe. She almost passed out and then you raped her.
“She was a virgin, something which you knew and something which you used to ensure her silence. You took her innocence to ensure her silence.”
Not the best wording, since having sex shouldn’t equate with guilt, but the point is that’s hardly the pleasantest way to stop being a virgin. The point is also that sex in fact is seen as guilt for a woman by many people, and that a raped virgin is seen as damaged goods, tainted, used up. In that sense the perp took her cleanness, her purity, her eligibility for marriage, and that he did it on purpose. Ugly.
This isn’t the standard kind of forced marriage though. In a way it seems like a bad idea to make this kind the first prosecution, because it’s not the paradigm.
(This is a syndicated post. Read the original at FreeThoughtBlogs.)
Karen James has good things to say on Twitter about this “phwoaaar women in the lab eh” bullshit. (That’s something Twitter is good for. Arguing about complicated subjects, no. Commenting on sexist or racist bullshit, yes.)
Karen James @kejames 6 hours ago
That Tim Hunt & others feel comfortable being overtly sexist in public says a lot about the larger environment in science.Brava @girlinterruptin on the larger problem around Tim Hunt’s remarks, this para especially. http://occamstypewriter.org/sylviamclain/2015/06/09/cry-cry-cry-for-backwards-nobel-laureates/ …
I dislike the workforce argument 4 why sexism & other isms are wrong. MT @royalsociety Science needs women http://ow.ly/O5t5c #wcsj2015
I had the same thought when I read the Royal Society’s statement. It annoyed me. Never mind the faff about “we need women in the labs” – say it’s shit.
The workforce argument suggests if we didn’t need ‘the research capabilities of the entire population’, sexism would be a-ok. @royalsociety
Erin @EmicAcademic 6h6 hours ago
@kejames @royalsociety Similar to “promote diversity=better profits!”; equality is only important when it is profitable for the ppl in powerIt’d be better if those trying to ‘distance themselves’ from misogyny would just say ‘sexism is wrong because it is wrong’. @royalsociety
Are they worried people will start talking about political correctness and identity politics? Or, worse, being pussy-whipped? Are they worried that sexism actually doesn’t matter unless it damages the bottom line?
Oh well. It’s only women.
(This is a syndicated post. Read the original at FreeThoughtBlogs.)
Is it something in the Bovril?
Cat Ferguson at BuzzFeed reports:
Tim Hunt, who won the 2001 Nobel Prize in medicine for his work on cell duplication, was speaking at an invitation-only lunch in honor of women in science. He reportedly opened his talk by saying: “Thanks to the women journalists for making lunch.”
The 72-year-old scientist went on to say that he has a reputation as a chauvinist, and that labs should be segregated by sex. The problem with female scientists? “You fall in love with them, they fall in love with you, and when you criticize them, they cry!”
“You” of course are a heterosexual male. Isn’t everyone? Everyone who counts?
Hunt is a member of the Royal Society, which quickly distanced itself from the remarks, first tweeting “Tim Hunt’s comments don’t reflect our views,” and then releasing an official statement.
“The Royal Society believes that in order to achieve everything that it can, science needs to make the best use of the research capabilities of the entire population,” they wrote on their blog.
“Too many talented individuals do not fulfill their scientific potential because of issues such as gender and the Society is committed to helping to put this right. Sir Tim Hunt was speaking as an individual and his reported comments in no way reflect the views of the Royal Society.”
Also, he’s obviously an asshole, and assholery is not part of the mission of the Royal Society.
(This is a syndicated post. Read the original at FreeThoughtBlogs.)
Sophie Elmhirst has a long profile of Richard Dawkins in the Guardian. It’s partly about his new career of creating uproars on Twitter, and whether or not that’s a good idea.
The two strands of Dawkins’s mission – promoting science, demolishing religion – are intended to be complementary. “If they are antagonistic to each other, that would be regrettable,” he said, “but I don’t see why they should be.” But antagonism is part of Dawkins’s daily life. “I suppose some of the passions that I show are more appropriate to a young man than somebody of my age.” Since his arrival on Twitter in 2008, his public pronouncements have become more combative – and, at times, flamboyantly irritable: “How dare you force your dopey unsubstantiated superstitions on innocent children too young to resist?,” he tweeted last June. “How DARE you?”
“Flamboyantly irritable” is a good way of putting it. There are problems with both, especially in a famous Oxford academic – and especially when they are irritable rather than witty or probing. Anybody can do irritable, and anybody does; it’s hard to see why Dawkins needs to join that massive and uninteresting crowd.
These days, Dawkins describes himself as “a communicator”. But depending on your point of view, he is also a hero, a heathen, or a liability. Many of his recent statements – on subjects ranging from the lack of Nobel prize-winning Muslim scientists to the “immorality” of failing to abort a foetus with Down’s syndrome – have sparked outraged responses (some of which Dawkins read aloud on a recent YouTube video, which perhaps won him back a few friends). For some, his controversial positions have started to undermine both his reputation as a scientist and his own anti-religious crusade. Friends who vigorously defend both his cause and his character worry that Dawkins might be at risk of self-sabotage. “He could be seriously damaging his long-term legacy,” the philosopher Daniel Dennett said of Dawkins’s public skirmishes. It is a legacy, Dennett believes, that should reflect the “masterpiece” that was The Selfish Gene and Dawkins’s major contribution to our understanding of life. As for Twitter: “I wish he wouldn’t do it,” Krauss said. “I told him that.”
Lots of people have told him that – friends and colleagues, I mean, not just onlookers.
Dawkins regularly goes on fundraising lecture tours, where his fame comes in useful. Tickets for a tour of the US in June – “an evening with Richard Dawkins”, in theatres in Portland, Oregon, Rochester, Minnesota and Boston – are on sale on the RDFRS website for $35. Access to a VIP reception beforehand is $250. Membership of the “Dawkins circle” costs from $1,000 to $9,999 a year, winning you discounts to the foundation’s online store, invitations to events with “RDFRS personalities” and, at its most expensive, two tickets for an “invitation-only” event with Dawkins himself. The fundraising is led by Robyn Blumner, the full time CEO of his foundation; Dawkins is her celebrity draw. “I’m totally hopeless at asking for money,” said Dawkins. “So I do work extremely hard at trying to be charming.”
Twitter not included.
For Dawkins, the science has always come first; his atheism is simply a natural extension of a lifelong quest to do Darwin’s work on Earth. As for the suggestion his public interventions over the past few years have done more harm than good – both to himself and his cause: “That does worry me,” Dawkins conceded, and yet he cannot quite resist the urge to wade in. “I think there is a curious desire in humans, maybe not all humans but certainly in me, to put things right,” he said. “There’s a joke in the New Yorker or something like that, of a man at a computer. It’s obviously very late and his wife is begging him to come to bed. He’s saying, ‘I can’t come to bed. Somebody’s wrong on the internet.’”
Twitter is not the best medium for putting things right. It’s one of the worst.
In recent years, the following sequence of events has become something of an online soap, regular and predictable: Dawkins tweets, is criticised for being deeply offensive, and then writes a long article to explain what he actually meant, which usually is not too far from what he said in the first place, but expressed with slightly more nuance. Since Dawkins joined Twitter seven years ago, he has amassed more than a million followers. He tweets assiduously, attracted by the medium’s limitations: “I’m sort of mildly intrigued by the art form of précising something into 140 characters; it’s not an easy thing to do. And there’s a certain satisfaction in the skill of doing it.”
Avoiding the obvious joke, I will make the less obvious point that the satisfaction fades pretty quickly, or at least it did in my case and I think probably in most people’s. You get the hang of it and then it just becomes a tool – it can be good for rapid conversations if the participants are witty enough, but no one tweet is likely to be a work of art. I think the medium’s limitations are something Dawkins shouldn’t be attracted by – I think they don’t work in his favor.
There was the pot of honey, for instance, as Elmhirst goes on to say.
Even on more serious topics, Dawkins cannot quite fathom how often he finds himself at the centre of online firestorms. “I do seem to be horribly susceptible to being misunderstood,” he said.
And why is that? If it’s a pattern, there’s probably a reason for the pattern. I think I know what it is.
“Quite a lot of what I do on Twitter is try to raise a discussion point,” he said. “It’s as though I was doing a seminar with students and said, ‘Here’s an interesting thought, X. What do you think about X?’” He is then mystified when his hypothesis is met by a chorus of criticism and abuse. “Very often I’m not making a point, but asking a question.” Sometimes his questions seem genuinely curious: “Whistling requires precise tongue positioning, like finger on violin string. Yet most can whistle tunes sans training. Interesting?” But often they are more rhetorical: “Truly? Is Sweden such a fatuously ridiculous country, bending over backwards to accommodate religious idiocy?”
Now I’m picturing fatuously ridiculous Sweden bending over backwards, and snickering.
Last July, Dawkins wrote, in 136 quickly infamous characters, “Date rape is bad. Stranger rape at knifepoint is worse. If you think that’s an endorsement of date rape, go away and learn how to think.” For Dawkins, this was simply the illustration of a basic point of logic; on the other hand, he was using a highly sensitive crime as an example. “If I used another example it would have been obvious,” Dawkins said, by way of explanation. “The point is there are people who seriously refuse to admit that some rapes are worse than others.” Isn’t that a judgment to be made by the person who’s experienced it? “Exactly, which is why I said date rape may be worse than stranger rape. I said that. It’s up to the victim to decide … But it’s absurd for the thought police to come along and say that it is forbidden to allow a woman to rank some rapes as worse than others … This is a logical point, and there are people who say that emotion trumps logic.” For Dawkins, the idea that someone could understand his argument and still disagree with him was bewildering. “There must be something wrong with how I’m expressing it,” he said. In the presence of his logic, there is no room for an alternative view.
When did he write that? Right after we issued the joint statement. Two days after, if I remember correctly.
Perhaps the greatest source of disquiet within the atheist movement – particularly in the US, where the movement, under the broad banner of “skepticism”, is more active and organised – is among feminists. Greta Christina, an American feminist and atheist blogger, first met Dawkins at an event in 2009. It was a fantasy made real. “He was the reason I started calling myself an atheist … [meeting him] was one of the proudest moments of my life.” Then, in 2011, Dawkins waded into a comment thread under a blogpost about a discussion of sexual harassment that had recently taken place at a skeptics’ conference in the US: “Dear Muslima,” Dawkins wrote to an imagined Muslim woman, “Stop whining, will you. Yes, yes, I know you had your genitals mutilated with a razor blade, and … yawn … don’t tell me yet again, I know you aren’t allowed to drive a car … But stop whining, will you. Think of the suffering your poor American sisters have to put up with.”
The attempt at satire went down badly: Dawkins appeared to be dismissing any concerns about sexual harassment (“He spoke some words to her. Just words,”) and doing so by ranking the experiences of women. He later apologised, but it marked, for Christina, a “disappointing and discouraging” turn for Dawkins, who had become, in her eyes, “so troubling, in such serious ways, and in particular so stubbornly troubling”.
Dawkins has always called himself a “passionate” feminist. As a fellow at New College, he agitated to allow women to be admitted, a change that occurred in 1979. “I show my feminism very largely in the Islamic context,” he said. “Because if women are having a hard time anywhere in the world, it’s there … I get impatient with American feminists who are so obsessed with being looked at inappropriately over the water cooler at work or whatever it is, that they forget that there are women being literally stoned to death for the crime of being raped.”
No, we don’t. We don’t forget. I, for instance, write about both. A lot.
His position has been interpreted in unfortunate ways by some of his followers. “Because he’s such a hero in the movement,” the American feminist Ophelia Benson said, “that gave a green light to an awful lot of people in the movement who thought it was okay to harass [feminists].” In recent years, online sceptic forums have been deluged with bilious anti-feminist posts and crude photoshopped images of women.
In an attempt to quell the increasingly unpleasant tone of discussion, Dawkins released a statement last August, jointly written with Benson, calling for an end to the online abuse. Dawkins added a personal footnote: “I’m told that some people think I tacitly endorse such things even if I don’t indulge in them. Needless to say, I’m horrified by that suggestion. Any person who tries to intimidate members of our community with threats or harassment is in no way my ally and is only weakening the atheist movement.”
A few weeks later he was back on Twitter writing comments about how a drunk woman’s evidence was unreliable in a rape trial. Why? “Because I not only care passionately about truth, I care passionately about justice.” (Should it not worry him more that such a tiny proportion of rape cases make it to court at all? “Oh absolutely … I care very passionately about that, of course I do.”) Benson, who had encouraged Dawkins to write the statement in the first place, looked on in despair. “No, no, Richard,” she remembered thinking. “That was not the idea.”
Yup. That is what I thought.
But don’t worry: the balance sheet comes out right.
“Ultimately, will his net impact be positive?” Krauss asked. “I think the answer’s yes. For all the intelligentsia and all the people who are offended, I see a much larger audience that I hadn’t appreciated for whom these issues are brand new.”
Again, I will avoid the obvious retorts. I’m tired of uttering them.
(This is a syndicated post. Read the original at FreeThoughtBlogs.)
Oh, do better, State Department. Come on.
Via Paul Fidalgo at The Morning Heresy – a passage from the daily press briefing at State.
QUESTION: Saudi Arabia.
MR RATHKE: Yeah.
QUESTION: Do you have any comment or reaction on the upholding by the supreme court of the blogger’s verdict and punishment by flogging?
MR RATHKE: We are deeply concerned that the Saudi supreme court has upheld the 10-year prison sentence and 1,000 lashes for human rights activist and blogger Raif Badawi for exercising his rights to freedom of expression and religion. As we had previously said back in January, the United States Government continues to call on Saudi authorities to cancel this brutal punishment and to review Badawi’s case and sentence. We strongly oppose laws, including apostasy laws, that restrict the exercise of freedom of expression, and we urge all countries to uphold these.
QUESTION: So would you like to see this – the court said the only way it could be overturned was with a royal pardon. Would you be – are you looking for the new king to grant a pardon in this case?
MR RATHKE: Well, I don’t have anything further to say about the internal workings of how Saudi authorities may address the case, but I would go back to our call on Saudi authorities to cancel this punishment and to review the case and review the sentence.
QUESTION: Well, that sounds to me like you’re calling for the king to pardon him.
MR RATHKE: I don’t have –
QUESTION: Well, if you called on them –
MR RATHKE: — more to say about –
QUESTION: — back in January to review the case and then to cancel the punishment, they have reviewed it now, the court has at least, and upheld it. So you still want it to be reviewed and – the case to be reviewed and the punishment to be canceled, correct? That’s what I’m hearing.
MR RATHKE: Yeah, that’s our answer.
QUESTION: The only way – the court says the only way that that can happen is if a royal pardon is issued. Ergo, or does that mean that you are calling on the king to issue a pardon?
MR RATHKE: I’m not going to go beyond what I said. That’s –
QUESTION: Well, then it doesn’t sound like – I mean, if you won’t call on the king to issue a pardon, which is what the court says is the only way that the punishment or the case can be dismissed, then I don’t understand what the point of you getting up here and saying that you’re deeply concerned about it is because you’re clearly not going to do anything – do the one thing that – or call on the king to do the one thing that –
MR RATHKE: To go back to the verb you used earlier, I’m not going to parse the Saudi court’s decision. But the United States Government’s view remains that we believe that the punishment should be canceled and that the case and the sentence should be reviewed.
QUESTION: But if the only way that that can happen is by royal pardon, why wouldn’t you call on the king to issue a royal pardon?
MR RATHKE: I just don’t have anything further to say on that one.
Thanks for nothing.
(This is a syndicated post. Read the original at FreeThoughtBlogs.)
A couple of people who dislike one or more of my recent posts have explained their thinking to me via…
…Twitter.
Why do people do that? What is the point? They could comment here, they could email me, they could (if they’re friends) talk to me on Facebook…but instead they choose the medium where you can write only 140 characters at a time.
Why?
It always fills me with a vast weariness when people do that.
Ok, now what? There are things I can say to each blurt, but what is the point? I don’t want to blurt. I want to be able to use however many characters I need for the purpose. I don’t want to engage in a dance of blurts.
So I just sigh and ignore the blurts.
Seriously, if you want to talk to me about something complicated, do it anywhere but Twitter. Twitter is the wrong damn medium for that.
(This is a syndicated post. Read the original at FreeThoughtBlogs.)
Originally a comment by Salty Current* on Living in the box [guest post by Seth].
There are so many other things going on here. Survival. Solidarity. Empathy. To me those are the keys… not making sure everyone is on the same page theoretically. Imagine one person saying, “I’m just trying to breathe here” and receiving the answer, “But what does that mean for ME?”
This strikes me as a strange argument. Taking into consideration how our actions affect others who are oppressed and struggling, listening to their concerns and taking them into account, is pretty much the definition of solidarity.
There was a great interview by Chris Hayes of Brittney Cooper several months ago, at the time the hidden-camera street harassment video went viral. I think he expected Cooper to basically side with those arguing that the video contributed to racial bias, if inadvertently, and leave it at that. Instead, she recognized the validity of that argument (and expanded it: the reception of the video also tended to sideline the vulnerability of black girls and women to street harassment); but then went on to suggest that black men, rather than focusing on this exclusively, could find their solidarity with women by seeing the problem within a shared framework of wanting to occupy public space and not be harassed – in their case, by police, in women’s, by men. It wasn’t a rhetorical game, but an accurate framing of the problems that could allow people to see their struggles in common terms rather than being set against each other.
It seems to me that this shared frame for solidarity has been missing from the current discussions, but it certainly exists. I’ve been arguing for a while that we can look at these struggles in terms of freedom, specifically freedom of self-definition and self-determination. The existential freedom to craft our identity and our own path rather than being chained to a false essence. The claim to that freedom unites all struggles for liberation, and those who oppose it with essentialist arguments, be they feminists, trans activists, or those hostile to these movements, are opposing liberation.
By the way, I think Lady Mondegreen’s suggestion on the other thread about asking some trans people who want to have a real discussion to write some posts was a good one. I think there are people who for whatever reason want to divide and sow hostility between our groups and movements, but that our goals are actually the same and we can better realize that through dialogue that focuses on what unites us rather than divides us. (And, in purely selfish terms, I’m sure I could learn a lot from it.)
*Yes two in one day. What can I say? She’s on a roll.
(This is a syndicated post. Read the original at FreeThoughtBlogs.)
Zinnia has a terrific post from last December explaining how and why trans people got shoved into hyper-gendered boxes.
When it comes to transitioning, many people seem to equate living as a woman with being stereotypically feminine. It’s a common assumption that trans women express their womanhood via conventional or even excessive femininity. Movies and TV shows often depict trans characters as far more feminine than most cis women – at times absurdly so. Tabloids focus on conventionally attractive models and actresses; when Christine Jorgensen became one of the first widely known trans women in 1952, front-page headlines described her as a “blonde beauty”.
Sound familiar? It sounds to me exactly like the reactions to Caitlyn Jenner’s Vanity Fair cover. It sounds exactly like that patronizing “ooooooh great job of being a gorgeous woman!!” commentary, as if that were the whole and only meaning of being a woman.
A 2002 study in Poland used a derivative of the Bem Sex-Role Inventory to evaluate 132 trans people and 438 cis people. Among the cis men, 4% were classified as feminine, 48% as masculine, 24% as androgynous, and 24% as undifferentiated. In comparison, trans men were more likely to be rated feminine, less likely to be masculine, and more likely to be androgynous. These results don’t really align with the suggestion that trans men exhibit stereotypical or excessive masculinity. And among cis women, 34% were rated feminine, 16% masculine, 28% androgynous, and 22% undifferentiated. While no trans women were classified as masculine, only 52% were rated feminine, with the remainder being androgynous or undifferentiated. Trans women were actually more likely to be rated androgynous than cis women.
A 2012 study in Spain used the inventory to examine 156 cis people and 121 trans people, with somewhat different results. Here, trans women were less likely to be rated feminine than cis women, and more likely to be rated androgynous and undifferentiated. Trans men were, again, more likely than cis men to be classified as feminine, and less likely to be masculine.
Neither of these studies supports the idea that trans people are any more extremely masculine or feminine than cis people. Instead, we see that trans people express their gender in diverse ways, much as cis people do.
So, she asks, why do the stereotypes persist?
To understand this, it’s necessary to look at the history of how gender dysphoria is defined and diagnosed.
In the 1950s and ‘60s, the medical community finally began to recognize gender dysphoria as a treatable condition. They now faced the questions of how to determine if a person is trans, and whether transition treatments are appropriate for them. At a time when the very idea of medical transition was widely unfamiliar to the public, therapists and doctors aimed to make the process seem legitimate and unchallenging to social norms.
How to do that? By “ensuring that any trans people who were accepted would conform closely to gender stereotypes.”
In a 1973 paper, Dr. Norman Fisk of the Stanford gender clinic listed certain factors pertaining to “the overall team decision as to acceptability for sex conversion”. Among these were “appreciation of core gender principles” and “physical passability” – the degree to which a trans woman was perceived as indistinguishable from a cis woman.
So what exactly were those “core gender principles”? A 1971 paper by Stoller contains a lengthy description of what he believed to be the defining features of trans women and trans men. As children, trans women are depicted as “developing a feminine gracefulness of movement”, drawing “beautiful women”, identifying with feminine women in television or movies, and enjoying “trying on jewelry and makeup”.
And so on.
Trans people had a powerful incentive to meet these clinical standards: their ability to transition was at stake. The problem, of course, was that these criteria were based on archaic gender norms. Women were expected to be feminine, conventionally attractive, interested in jewelry, straight, emotional, lacking sexual interest, and married to men. Men were expected to be masculine, interested in sports and construction, and take straight women as partners. Dr. Fisk actually stated that the Stanford program offered “grooming clinics where role-appropriate behaviors are taught, explained and practiced”.
In short, these clinics seemingly aimed to produce only people who were as stereotypical in their gender as possible, perhaps not realizing that cis women may also be tomboyish, sexually outgoing, or attracted to women. There is evidence that this kind of selection process is still occurring: a 2004 study of 325 trans people seeking treatment in the Netherlands found that patients were more likely to be referred for hormone therapy when their appearance was perceived to align more closely with their gender.
Boxes boxes boxes. Rigid hard immovable boxes.
Cis people set these stereotypical standards. We conformed to them against our own inclinations. And cis people now have an entrenched stereotype of us as overly feminine. Well, whose fault is that?
I’m gonna guess cis people’s?
For trans women, transitioning tends to involve a reduction in attributes perceived as male, and an increase in attributes perceived as female. On our own, we can grow our hair out, change the way we dress, and practice altering our voice and how we walk. Medical treatment can change our facial appearance, give us a more feminine body shape, reduce our body hair, and enhance our breast growth. If the Kessler and McKenna study on gender cues is applicable to everyday life, this suggests a newfound abundance of female cues could mean we don’t have to pay as much attention to maintaining all of them. Once many of them are solidly in place, it might start to feel less like we have to push our gender cues to the maximum.
In my experience, the difference has been substantial. Before, I felt like I was walking a tightrope, constantly making sure my presentation was in perfect balance to avoid being misgendered. But after two years of transitioning, I’ve realized that I just don’t care – and now, neither does anyone else. Nowadays, makeup is a rare indulgence. I’ve shaved half my hair off because I just felt like it. I don’t need padded bras anymore, and I don’t usually bother with bras at all. I have a huge trans tattoo on my chest. For me, transitioning didn’t mean turning into Bree from Transamerica – I’m more like some kind of frumpy dubstep housewife. That’s because my gender is finally for me, not for everyone else.
I used to get mistaken for a man occasionally – but the difference is it didn’t matter. That was my privilege.
[This is a long and brilliant piece, with illustrations; read the whole thing.]
(This is a syndicated post. Read the original at FreeThoughtBlogs.)